Our scientific focus
The technical challenge of safely and effectively delivering nucleic acid payloads to therapeutically relevant areas of the body has been a major obstacle to unlocking the enormous potential of nucleic acid medicines. Genevant scientists have operated at the forefront of the field for nearly 20 years, revolutionizing lipid nanoparticle composition, formulation and manufacture. Biodegradable lipids (to reduce lipid accumulation without affecting potency), lyophilized (freeze dried) drug product (to improve supply chain efficiency) and access to cells and tissues outside the liver are just some of the ways we set the pace.
Our unique capabilities
Companies in the nucleic acid space typically work with only a singular modality, such as mRNA, siRNA or gene editors. Our multiple platforms provide the ability to exploit multiple constructs, and our technology is validated by the field-based licenses that we have granted to industry leaders.
Our platforms
Genevant’s scientific leadership has pioneered Lipid Nanoparticle (LNP) nucleic acid delivery for 20+ years. With our proprietary advantages, LNPs can provide both optimal uptake into desired cells and efficient release, resulting in functional delivery of nucleic acid payloads to target tissues. Our LNP platform, the delivery technology behind the first and only siRNA-LNP product to achieve regulatory approval (Alnylam’s ONPATTRO®), also enables a wide array of mRNA-based applications, including vaccines, therapeutic protein production, and gene editing.
Genevant’s LNP platform is the most clinically validated in the space, with experience in more than 600 subjects across more than a dozen discrete product candidates — including in the antiviral, oncology and metabolic disorder therapeutic areas. And our manufacturing process has been scaled to support future commercialization, with batch sizes of up to a kilogram of nucleic acid.
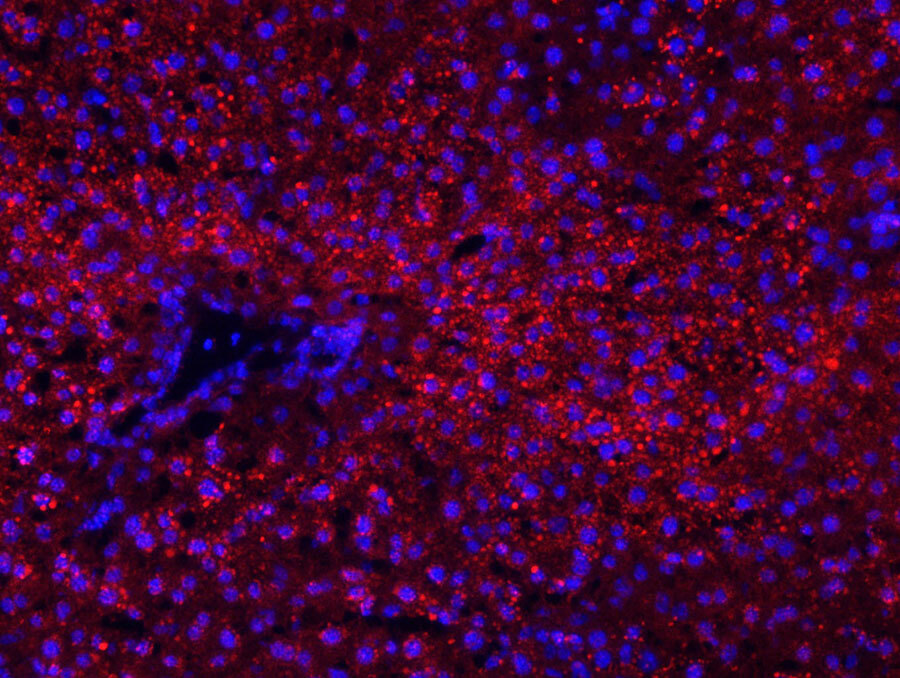
Optimizing a delivery solution
LNPs can be engineered to exhibit favorable pharmacologic properties, such as potency, dose responsiveness (including repeat-dose efficacy), rapid lipid metabolism, and tolerability, and can enable stable drug product for dosing at therapeutically relevant levels. Applying our knowledge of structure-activity relationships and design principles, we have extended our LNP technology for delivery to extrahepatic tissues, such as lung and stellate cells.

Our scientists bring expertise in lipid chemistry, formulation development, in vivo pharmacology, pharmaceutical development, scale up and technology transfer for GMP manufacture, and regulatory affairs and have helped collaborative partners progress several diverse programs into clinical trials, including both siRNA and mRNA payloads and multiple routes of administration – often within 12 months from lead selection.

Constant innovation
Our Ligand Conjugate Delivery Platform enables functional delivery of small interfering RNA (siRNA) molecules to disrupt the production of proteins implicated in human disease.
Our platform connects ligands with high selectivity and strong binding affinity for target receptors to siRNA oligonucleotides via unique linkers. Our ligands include multivalent N-acetylgalactosamine (GalNAc) “motifs” for delivery to hepatocytes (liver cells) and other novel structures for extrahepatic organs, cell types or tissues such as hepatic stellate cells, tumors and macrophages. Beyond the ligand conjugates, Genevant has substantial experience designing siRNA, for LNP as well as ligand and conjugates, employing a range of chemical modifications to confer optimal potency, pharmacokinetics and tolerability. Our GalNAc technology has been scaled up to support various clinical trials, with promising results.
One of the principal hurdles for conjugate-based nucleic acid delivery is endosomal entrapment, whereby the siRNA is “trapped” in intracellular compartments (endosomes) before being destroyed, in many cases abrogating potency entirely. We are developing our next generation ligand conjugate platform to enable unique endosomal release functionality. In testing to date (nonhuman primates), we have shown a potency advantage compared with standard GalNAc conjugate by an order of magnitude, as well as a more rapid onset of activity and longer duration of effect.
Contact us
We are always ready to connect with people interested in the pioneering work we do.
